Maker Lab Research

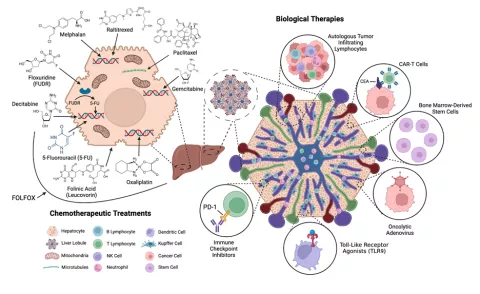
My lab research focuses on tumor immunology and early cancer detection. During my post-doctorate fellowship in the laboratory of Dr. Steven Rosenberg in the Surgery Branch of the NIH/NCI I authored many of the initial phase I/II trials utilizing anti-CTLA4 antibodies. After that I worked on expanding the role of immunotherapy for gastrointestinal tumors. My NCI/NIH and DoD-funded research program has identified an immunostimulatory cytokine capable of activating and supporting the proliferation of antigen-specific T-cells to incite an anti-tumor immune response in colorectal liver metastases. This strategy is currently being investigated in combination with oncolytic viruses and immune checkpoint blockade to elicit complete tumor responses. Furthermore, my lab has developed a gene signature to predict malignancy in IPMN from pancreatic cyst fluid. We house the international IPMN cyst fluid repository, one of the largest biobanks of its kind, for which he serves as the international PI. With this collaborative, we are currently validating a single-platform biosignature to accurately predict the malignant potential of pancreatic cysts.